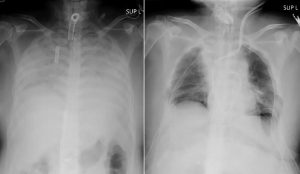
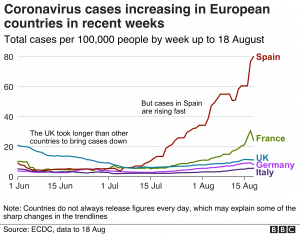
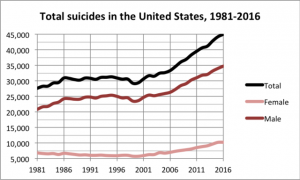
Dr. According to Randeep Guleria, Covaxin’s trial run for kids has gone beyond 2/3 steps. The information available from there is that the vaccine will be available in September. Expecting. Approval will be available that month.
Read more: Corona Delta Plus species cause new concern in India! Vaccines will not be able to stop
He also mentioned the Pfizer-BioNTech vaccine. According to him, if this vaccine is also exempted, then two vaccines will be available for children. From June 6, Delhi AIIMS started clinical trials of the vaccine in children. Children from 2 to 18 years of age are participating in this trial run. In May, Drugs Controller General of India (DCGI) authorized India Biotech to start Covaxin trial run.
Incidentally, the third wave of the corona is inevitable. It may hit the country in the next 6-7 weeks. AIIMS chief Dr Randeep Guleria said this adding to the panic.
He added that the only way to avoid maximum damage from the third wave was to increase vaccination rates. However, he acknowledged that in a country with such a large population, it is extremely challenging to vaccinate all people. AIIMS chief Dr Randeep Guleria has even expressed concern over the Delta Plus species of corona virus.